Flow cytometry : Technology for cell analysis
- October 25,2020
- 20 Min Read
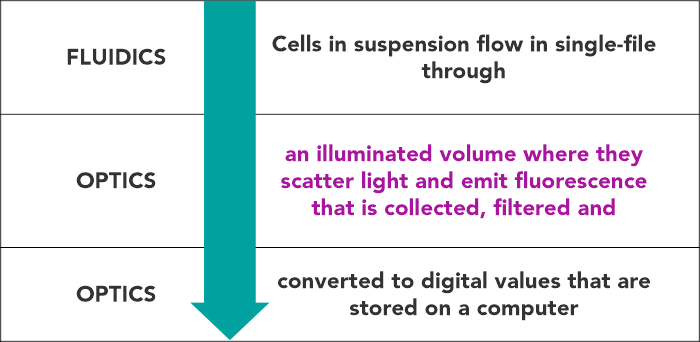
Flow cytometry is a technology that simultaneously measures, and then analyses multiple physical characteristics of single particles, usually cells, as they flow in a fluid stream through a beam of light, usually a laser.
WHAT CAN A FLOW CYTOMETER TELL US ABOUT A CELL?
- Its relative size
- Its relative granularity or internal complexity
- Its relative fluorescence intensity
Samples are prepared for fluorescence intensity measurement through transfection and expression of fluorescent proteins, staining with fluorescent dyes or immunostaining with fluorescently conjugated antibodies.
WHAT ARE THE COMPONENTS OF A FLOW CYTOMETER?
 WHAT CAN A FLOW CYTOMETER BE USED FOR?
WHAT CAN A FLOW CYTOMETER BE USED FOR?
Flow cytometry is a powerful investigative tool with applications in multiple disciplines such as immunology, hematology, virology, molecular biology, cancer biology, and infectious disease monitoring.
-
Immunophenotyping
- Most leucocytes have specific cluster of differentiation (CD) antigens or markers that are associated with the lineage and maturation stage of these cells, and help in defining them as distinct populations of cells.(1)
- Flow cytometry is most commonly used for immunophenotyping of hematopoietic cells– especially useful in the diagnosis of hematologic malignancies. In flow cytometric immunophenotyping, we label the cells, usually the hemopoietic cells with fluorochrome-tagged antibodies directed against specific CD markers.(1)
- A flow cytometer simultaneously analyzes mixed populations of neoplastic and normal cells using multiple cellular parameters and provides composite and valuable information about the cells of interest. This in turn helps in 2 characterization and identification of these cells, and in distinguishing normal cells from the neoplastic cells. (1) This approach is commonly followed in the diagnosis of hematolymphoid neoplasms.
- In addition to lineage markers that define populations of hematopoietic cells, other markers are used to characterize each cell population. These markers can include activation markers, memory markers, tissue homing markers and chemokine receptor markers. Immunophenotyping can also include intracellular markers such as Immunoglobulins and their light chains, FoxP3 (defines Treg cells), cytokines, proliferation markers, and antigen specific markers.(1)
- Several other common examples of flow cytometric immunophenotyping in hematology are given below:
- Enumeration of lymphocyte subsets: Flow cytometry is extensively used for enumeration of T- and B-lymphocyte subsets, especially, CD3, CD4, CD8, CD19, CD16 and CD56 positive cells for assessment of inherited and acquired immunodeficiency states, e.g. HIV infection. There is renewed interest in the status of these subsets in COVID-19 infection.(2–4)
- HLA B27 typing is another highly sought after clinically useful test involving flow cytometry. This test is used for the diagnosis of ankylosing spondylosis and for its distinction from the other seronegative arthritis. (2–4)
- Diagnosis of Paroxysmal Nocturnal Hemoglobinuria (PNH) using immunophenotyping of red cells and white cells has been one of the emerging areas in clinical application of flow cytometry. Flow cytometry allows identification of very small populations of PNH cells in circulation.(2–4)
- ABO and Rh grouping of red cells: Flow cytometry has been found especially useful in resolving complex grouping problems and in bone marrow transplantation.(2–4)
- Cross matching in transplantation and post-transplantation monitoring of immune parameters.(2–4)
- Measurement of circulating fetal red cells in the maternal blood for assessment of feto-maternal hemorrhage.(2–4)
- Assessment of platelet function: Cases of suspected platelet function disorders that cannot be resolved after platelet aggregometry and other functional assays can be characterized further by flow cytometry.(2–4)
NON-IMMUNOPHENOTYPING APPLICATIONS
-
Measurement of apoptosis markers
- Rapid and quantitative measurement of apoptotic cells can be detected by flow cytometry.(4)
- Many different flow cytometric methods for the assessment of apoptosis in cells have been described including:
- Cell viability measurement(4)
- Detection of plasma membrane changes(4)
- Detection of active caspase-3 activity(4)
- Detection of mitochondrial proteins(4)
- DNA fragmentation(4)
-
Intracellular and circulating cytokine analysis
- Intracellular cytokine analysis is performed by treating immune cells with a protein transport inhibitor for 2 to 12 hours to allow for cytokines produced by the cells to accumulate within the cell, enabling better detection.(1)
- Following protein transport inhibitor treatment, cells are stained for viability markers and cell surface markers, then fixed and permeabilized for intracellular staining with anti-cytokine antibodies.(1)
- Micro-bead array systems are available for measuring the circulating levels of cytokines by flow cytometry.(1)
-
Proliferation analysis
- Cell proliferation can be measured by flow cytometry using several different assays and markers.(1)
-
Cell cycle analysis
- Cell cycle analysis is one of the pioneering applications of flow cytometry. During the cell cycle there are alterations in the genetic content, which can be observed by fluorescent dye tags, such as propidium iodide which bind to the DNA in a 1:1 ratio and provide quantitative data.(3) A normal DNA index (DI) indicates normal chromosomal content of the cells. In malignancies with hyperdiploid and hypodiploid chromosomes the DI correspondingly increases and 3 decreases. High ‘S’ phase of tumour cells suggests their high proliferative potential.(3)
-
Cell sorting
- Cell sorting utilizes a flow cytometer with cell sorting capabilities to separate and purify cells or particles for further analysis. Essentially, any cell or particle that can be made fluorescent can be separated by a cell sorter.(1)
-
Absolute cell counting
- The procedure utilizes fluorescent beads of a known concentration that is acquired along with the sample. The sample is analyzed and the number of cells is compared with the number of beads acquired in the same sample to generate the number of cells per milliliter.(1) This principle is used for lymphocyte subset enumeration.
-
Phagocytosis assays
- Using fluorescently tagged bioparticles or bacteria, it is possible to detect phagocytosis using flow cytometry.(1)
References:
McKinnon KM. Flow Cytometry: An Overview. Curr Protoc Immunol [Internet]. 2018 Jan [cited 2020 Oct 10];120(1). Available
from: https://onlinelibrary.wiley.com/doi/abs/10.1002/cpim.40
Nguyen DT, Diamond LW, Braylan RC. Flow cytometry in hematopathology: a visual approach to data analysis and interpretation
[Internet]. Totowa, NJ: Humana Press; 2003 [cited 2020 Oct 16]. Available from: http://site.ebrary.com/id/10181487
Bajgelman MC. Principles and applications of flow cytometry. In: Data Processing Handbook for Complex Biological Data
Sources [Internet]. Elsevier; 2019 [cited 2020 Oct 10]. p. 119–24. Available from: https://linkinghub.elsevier.com/retrieve/pii/B9780128165485000083
Adan A, Alizada G, Kiraz Y, Baran Y, Nalbant A. Flow cytometry: basic principles and applications. Crit Rev Biotechnol. 2017 Feb 17;37(2):163–76.
Want to book a test? Fill up the details & get a callback
Most Viewed
Premarital Health Screening
- 20 Min Read
Typhoid - Signs and Symptoms
- 3 Min Read
Home Isolation Guidelines - Covid-19 Care
- 5 Min Read
HLA B27 Detection: Flow Cytometry & PCR
- 1 Min Read