Types of tests for HIV & Monitoring (Suburban Medical Journal)
- December 22,2021
- 30 Min Read

THE HIV VIRUS – ORIGIN & TYPES
HIV is subdivided into two types, HIV-1 and HIV-2.
HIV-1 is further divided into three main groups; major (M), outliers (O) and new groups (N).
HIV-1 group M has a worldwide distribution HIV-2 is endemic in West Africa and has spread in the last decade to India and Europe. (1)
HIV-2 is less pathogenic than HIV-1, which results in a prolonged period until the signs of immunodeficiency and AIDS develop, and a mother-to-child transmission rate of HIV-2 is around 2% to 7%, compared to 10% – 40% in those infected with HIV-1.(1)
TYPES OF HIV DIAGNOSTIC TESTS
Types of tests for HIV diagnosis can be broadly classified into:
· Antigen-antibody based assays – 4 generations of tests available
· Nucleic acid amplification assays: HIV RNA based
o Polymerase chain reaction based assays
o Isothermal amplification based assays
o Loop mediated isothermal amplification based assays
o Recombinase polymerase amplification based assays
WHEN CAN HIV BE DETECTED?
To answer this question, we have to first reconcile with the concepts of eclipse period and window period and also the various antigens and antibodies that are the target of HIV assays.
Eclipse period:
After an exposure that leads to infection, there is a variable amount of time called the eclipse period in which no existing diagnostic test is capable of detecting HIV – this is known as the eclipse period (Figure1).(2)
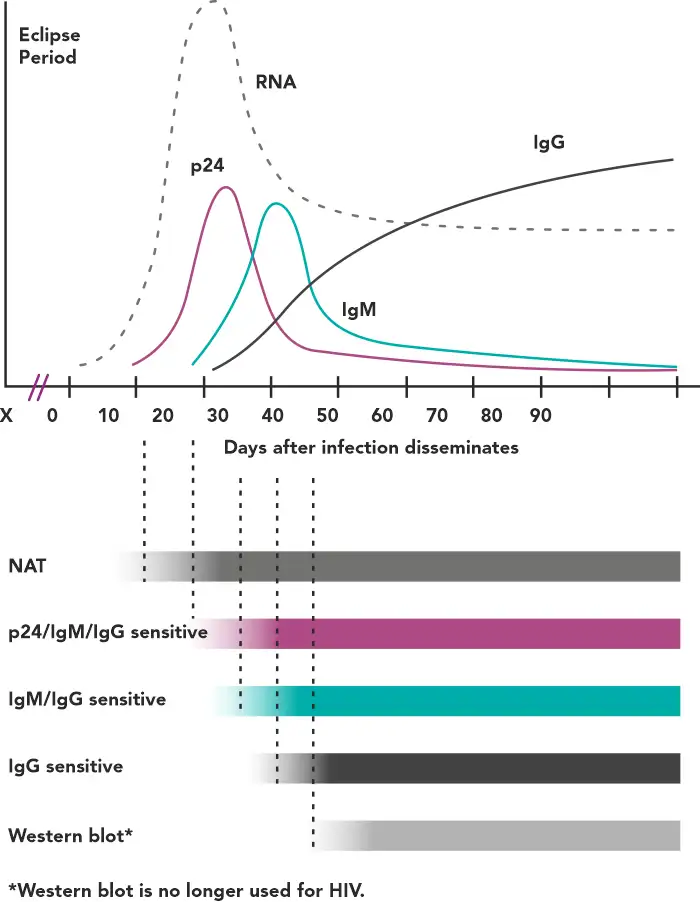
Figure 1 - Timeline of virological and serological events following an HIV infection(2)
Window period:
The time period between infection and the first reactive result on a given test is called the window period, the length of which depends on the target being detected by a particular assay. For example, tests capable of identifying anti-HIV IgM have a shorter window period compared with those detecting only IgG.(2)
GENERATIONS OF ANTIBODY/ANTIGEN TESTS FOR HIV DIAGNOSIS
HIV antibody/antigen tests have been typically classified into generations - each improving incrementally on its predecessor(s) in terms of performance and shortening of the window period.(2)
Table 1 - Generation of anti-HIV antibody tests(3–5)
The median window period for 4th generation tests is 17.8 days, whereas it is 23.1 days for the 3rd generation assays.(6)
There are some tests which are informally called 5th generation tests, but are not officially recognized as such because there is no reduction of window period from the 4th generation. These tests can also distinguish between samples positive for p24 antigen and for antibodies, and also between HIV-1 and HIV-2.(7)
NACO TESTING GUIDELINES – TESTING ALGORITHMS
Diagnosing HIV in asymptomatic patients(3):
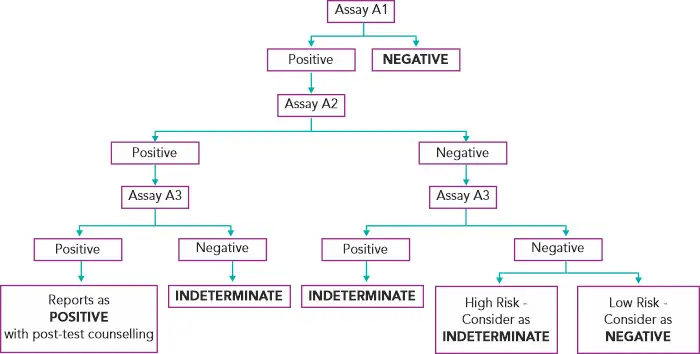
Assays A1, A2, A3 represent 3 different assays based on different principles or different antigenic compositions. Assay A1 should be of high sensitivity and A2 and A3 should be of high specificity. A2 & A3 should also, ideally, be able to differentiate between HIV- 1 & 2 infection.(3)
In case of an indeterminate result, testing should be repeated on a second specimen taken after 14 days to 28 days. In case the serological results continue to be indeterminate, then the specimen is to be subjected to PCR if facilities are available or refer to the National Reference Laboratory for further testing.(3) If the HIV-1 PCR is positive, it is confirmed as positive. If negative, do HIV-2 PCR testing to confirm or rule out HIV-2.
Diagnosing HIV in a patient with AIDS-indicator-disease symptoms(3):
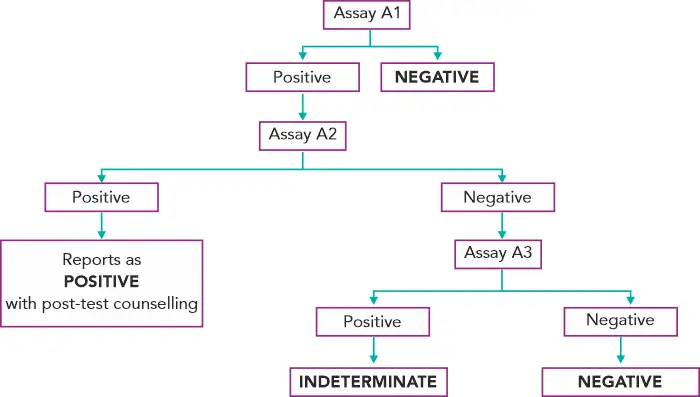
Diagnosis of HIV in infants with HIV positive mothers
HIV seropositivity in a child younger than 18 months is not diagnostic (because a positive antibody test result during the first 18 months of life could represent either persistent maternal antibody in an HIV–uninfected child or antibody newly produced by an HIV–infected child). Ergo, serologic testing of a child younger than 18 months cannot be used to diagnose HIV infection and virologic assays are required. However, a positive antibody test result in the infant generally indicates maternal HIV infection.(8)
It has been recommended that diagnostic testing with HIV RNA assays be performed(8):
· within the first 14 days of life, AND
· at 1 to 2 months of age, AND
· at 3 to 6 months of age.
If any of these test results are positive, repeat testing is recommended to confirm the diagnosis of HIV infection. A diagnosis of HIV infection can be made on the basis of 2 separate positive HIV RNA assay results. (8)
SUPERINFECTION
Superinfection of an HIV-infected person with a newly acquired HIV, e.g. by sexual transmission, has been observed frequently. The immunity established by the first infecting HIV strain remains incomplete due to viral mutation, which is in turn due to a lack of efficiently neutralizing antibodies and primed CD8 lymphocytes incapable of killing all HIV infected cells, especially those in the sanctuary sites. Thus, a multi-drug-resistant strain is also able to superinfect an HIV-infected patient, and as a result, a multi-drug-resistant HIV profile may be seen during follow-up in a patient who previously had a virus with a susceptible drug profile.(1)
OPPORTUNISTIC INFECTIONS
In individuals with chronic HIV infection not on treatment with antiretroviral agents, as the CD4+ count drops they are vulnerable to a multitude of infections which rarely occur in an immunocompetent host, hence the term opportunistic infections (OIs).(9)
Opportunistic infections are manifested depending on the severity of the suppression of CD4+ counts(9):
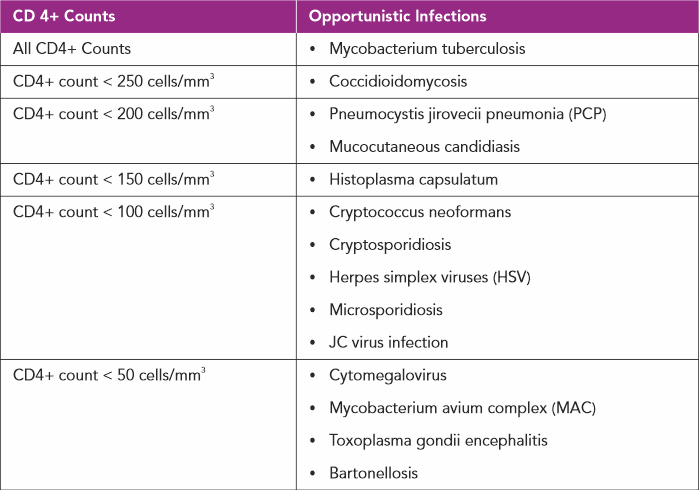
CO-EXISTING INFECTIONS WITH HIV
Some infections are known to co-exist with HIV(9): Syphilis, Human papillomavirus infection, Hepatitis B & C virus infection.
EVALUATION OF OPPORTUNISTIC & CO-INFECTIONS
In all HIV infected patients, regular monitoring of CD4+ cell counts is necessary for the early diagnosis and treatment of opportunistic infections. CD4+ counts also help in the initiation of prophylactic treatment regimens in the appropriate patients. Infection-specific diagnostic tests are listed below:
Mycobacterium Tuberculosis(10):
· All HIV positive patients require testing for latent TB infection (LTBI).
· The two current methods available for detection of M. tuberculosis infection in the United States, IGRA and TST, help differentiate infected from uninfected people.
· However, diagnostic accuracy of TST and IGRA are limited; a negative test does not exclude the diagnosis of LTBI or TB disease, and a positive test does not in itself mean LTBI therapy is warranted.
· Tuberculin skin test (TST) is performed and considered positive when there is a greater than 5 mm induration at 48 to 72 hours in HIV positive patients, without any clinical or radiologic signs of TB disease.
· Interferon-gamma release assays (IGRA) are FDA approved, consistent, and have higher specificity (92% to 97%) compared to (56% to 95%) for TST.
· An incidental finding of fibrotic lesions on chest X-ray should prompt further diagnostic testing for LTBI and active infection. The test of choice for active infection is the Xpert MTB/RIF Assay.
· In HIV positive individuals whose CD4+ counts are less than 200 cells/mm3, the presence of fibrotic lesions on chest X-ray which are highly suggestive of TB infection must be considered as having an active infection and subsequently, should be started on empiric treatment while performing further testing and awaiting results.
Coccidioidomycosis(10):
· Isolation of the organism on culture is part of the diagnosis, and so is the visualization of the spherules on histopathologic evaluation of the affected tissue.
· CSF cultures demonstrate positive results in less than 30% of patients with meningitis.
· Coccidioidal IgG and IgM serology are seldom positive in individuals with low CD4+ counts.
· Blood cultures are generally positive in diffuse pulmonary involvement.
Pneumocystis Jirovecii Pneumonia(10):
· Demonstration of the organism in tissue, bronchoalveolar lavage fluid or induced sputum samples is necessary for the diagnosis.
· Beta-D-glucan is found in fungal cell walls could be elevated in patients with PCP; however, needs further evaluation of both the sensitivity and specificity.
Mucocutaneous Candidiasis(10):
· Oropharyngeal candidiasis are diagnosed clinically based on the appearance and the ability to scrape off the white plaques. These lesions can be visualized using a potassium hydroxide (KOH) preparation which reveals yeast forms on microscopy.
· Endoscopy is necessary to diagnose esophageal candidiasis coupled with the demonstration of Candida yeast forms in tissues along with the isolation of candida in cultures.
· Vulvovaginal candidiasis can be diagnosed clinically followed by the demonstration of yeast forms using KOH preparation.
Histoplasma Capsulatum(10):
· Disseminated histoplasmosis is diagnosable by detecting histoplasma antigen in blood or urine. However, it is not sensitive to pulmonary infections.
· In patients with severe disseminated histoplasmosis, peripheral blood smears can show the organisms engulfed by white blood cells.
· Histopathological examination of biopsy material from involved tissues often demonstrate the characteristic 2µm to 4 µm in diameter budding yeast cells.
· The organism grows slowly and hence requires weeks for culture isolation; They have been cultured from bone marrow, blood, respiratory secretions in greater than 85% of AIDS patients.
· CSF cultures are rarely positive, and hence the diagnosis of meningitis is difficult. The usual cerebrospinal fluid (CSF) findings are lymphocytic pleocytosis, elevated protein, and low glucose. Fungal stains are usually negative, and CSF cultures are positive in a minority of cases.
Cryptococcus neoformans(10):
· Analysis of cerebrospinal fluid (CSF) generally demonstrates mildly elevated protein levels, low-to-normal glucose concentrations, and a variable presence of pleocytosis consisting mostly of lymphocytes.
· A Gram stain or an India ink preparation, if available, may demonstrate numerous yeast forms.
· In patients with meningitis or meningoencephalitis, cryptococcal antigen is easily detected in the CSF, as well as in the serum. Positive blood cultures present in up to 75% of HIV positive patients with cryptococcal meningitis.
· The BioFire FilmArray Meningitis/Encephalitis Panel PCR assay tests for many targets, including C. neoformans and C. gattii, and performs well in infections with a moderate to high fungal burden
Cryptosporidiosis(10):
· Diagnosis of cryptosporidiosis has traditionally been made by microscopic identification of the oocysts in stool with acid-fast staining or direct immunofluorescence, which offers higher sensitivity. Concentration methods (e.g., formalin-ethyl acetate) may facilitate diagnosis of cryptosporidiosis.
· Multiplex molecular methods are increasingly used for diagnosis, and can identify a greater number of cases than microscopic methods.
· A single stool specimen is usually adequate to diagnose cryptosporidiosis in individuals with profuse diarrheal illness, whereas repeat stool sampling is recommended for those with milder disease.
Herpes simplex viruses (HSV)(10):
· HSV DNA PCR is utilized for the detection of mucocutaneous lesions. All genital lesions must undergo typing as HSV-2 more commonly correlates with genital lesions than HSV-1.
· Type-specific serologic assays are commercially available and can be used for diagnosis of HSV-2 infection in asymptomatic individuals or those with atypical lesions. Type-specific serologic screening for HSV-2 for persons with HIV infection can be considered.
Microsporidiosis(10):
· These microsporidia can be detected by light microscopy using stains such as chromotrope 2R, calcofluor white and uvitex 2B which can help differentiate between the spores of the microsporidia and the cells and debris (e.g., stool). In patients with gastrointestinal symptoms, if the stool testing is negative and the infection is suspected, small bowel biopsy must be considered.
· In gastrointestinal disease, examination of three stools is often sufficient for diagnosis. If stool examination is negative and microsporidiosis is suspected, a small bowel biopsy may be useful.
· In biopsy specimens, microsporidia can be visualized with Giemsa, tissue gram stains (Brown-Hopps gram stain), calcofluor white or uvitex 2B (fluorescent brighteners) staining, Warthin-Starry silver staining, or chromotrope 2R.
JC virus infection(10):
· Progressive multifocal leukoencephalopathy (PML) diagnosis is by clinical and neuroradiologic clues.
· MRI findings of white matter lesions which are hyperintense (bright) on T2-weighted images and hypointense (dark) on T1-weighted sequences, in areas corresponding to the clinical deficits present in most cases.
· PCR can be utilized to detect JCV DNA in CSF.
· Perivascular mononuclear inflammatory infiltration present on histopathology.
Cytomegalovirus(10):
· Detection of CMV viremia by PCR occurs in both end-organ disease and without an end-organ state.
· A negative IgG level helps to look for an alternate etiology for symptoms.
· CMV retinitis diagnosis occurs with the help of an ophthalmoscopic examination which has a positive predictive value of 95%.
· CMV colitis is diagnosed by the demonstration of mucosal ulcerations on endoscopy or colonoscopy. Biopsy of these lesions shows characteristic intranuclear and intracytoplasmic inclusions on the histopathologic exam.
· In patients with CD4+ counts under 50 cells/mm^3, a regular fundoscopic exam is a requirement.
· The diagnosis of CMV pneumonitis requires consistent clinical and radiological findings (i.e., diffuse pulmonary interstitial infiltrates, fever, and cough or dyspnea), identification of multiple CMV inclusion bodies in lung tissue or cytology, and the absence of any other pathogens that are more commonly associated with pneumonitis. Detection of CMV in the lungs in the absence of these criteria typically represents shedding, rather than clinical disease.
Mycobacterium avium complex (MAC)(10):
· Growth of MAC from cultures of blood, lymph node, or any other sterile tissues or body fluids along with the clinical signs and symptoms are useful in the diagnosis of disseminated MAC infection.
Toxoplasma gondii encephalitis (TE)(10):
· Detection of anti-toxoplasma immunoglobulins G antibodies is noted in a majority of HIV positive patients with TE.
· Furthermore, a combination of clinical signs with imaging findings on CT or MRI of mass lesions, coupled with the demonstration of the organisms on brain biopsy is necessary for a definitive diagnosis.
Bartonellosis(10):
· Diagnosis of bacillary angiomatosis (BA) can be confirmed by histopathologic examination of biopsied tissue.
· BA lesions are characterized by vascular proliferation, and a modified silver stain (such as Warthin-Starry stain) usually demonstrates numerous bacilli. Gram stain and acid-fast staining are negative and not helpful.
· In immunocompetent patients, anti-bartonella antibodies might not be detectable for 6 weeks after acute infection; in contrast, by the time bartonella infection is suspected in patients with late-stage HIV infection, they usually have been infected with bartonella for months or even >1 year.
· As many as 25% of bartonella culture-positive patients never develop antibodies in the setting of advanced HIV infection.
Syphilis(10):
· A presumptive serologic diagnosis of syphilis is possible based upon non-treponemal tests (i.e., Venereal Disease Research Laboratory [VDRL] and rapid plasma reagin [RPR]) and treponemal tests (i.e., fluorescent treponemal antibody absorbed [FTA-ABS], T. pallidum particle agglutination [TP-PA], enzyme immunoassays [EIAs], chemiluminescence immunoassays [CIA], immunoblots, and rapid treponemal assays).
· Neurosyphilis is diagnosed by CSF examination which may show mononuclear pleocytosis, reactive CSF-VDRL or normal to mildly elevated protein concentration.
Human papillomavirus infection(10):
· Visual inspection of the warts is usually sufficient to make the diagnosis, although confirmation is obtained via biopsy.
· There is currently no role for HPV testing in HIV positive women. In these patients, if their cervical Pap test shows ASCUS, ASC-H, LSIL, HSIL they require referral for colposcopy.
Hepatitis B virus infection(10):
· All HIV positive individuals require testing for HBV infection.
· Testing, specifically, for hepatitis B surface antigen (HBsAg), hepatitis B core antibody (anti-HBc total), and hepatitis B surface antibody (anti-HBs) is necessary to identify those who have received preventative vaccination or in the diagnosis of chronic hepatitis B.
· In patients with chronic HBV, HBeAg, anti-HBe and HBV DNA must be tested to evaluate for the levels of replicating virus and progression of liver disease, progression to HCC and responses to therapy, respectively.
Hepatitis C virus infection(10):
· Detection of anti-HCV in blood is necessary for all patients with HIV.
· Qualitative or quantitative testing for plasma HCV RNA usually confirms chronic infection.
· Liver biopsy is reserved for the evaluation of HCV-related complications like fibrosis, assess prognosis and help with treatment options.
ASSAYS FOR HIV DISEASE MANAGEMENT
Laboratory testing in people living with HIV infection generally includes CD4 cell count (a marker of immune function) and HIV viral load assays. The HIV viral load should become undetectable during the course of treatment. Initiation of antiretroviral therapy is warranted regardless of CD4 cell count or viral load.
CD4+ Cell Count:
HIV has a particular tropism for cells with the CD4 surface protein, to which it binds with the gp120 envelope glycoprotein.
CD4 cells are lost through numerous mechanisms. HIV itself is directly cytotoxic to T cells, but cell death also occurs as part of the natural immune response and cell activation that occurs with any chronic infection. In the case of HIV, however, T-cell replacement from the thymus is adversely affected, so an increased T-cell turnover is not compensated for by a concomitant increase in production. CD8 cells are relatively spared, leading to a reversal of the usual CD4/CD8 ratio.
Current guidelines suggest that CD4 cell counts should be performed annually (or optionally) in patients who have been on antiretroviral therapy for at least 2 years, have undetectable viral loads, and have CD4 cell counts of 500/µL or more. In general, HIV viral load testing provides a better measure of resistance and noncompliance with treatment.(11)
HIV Viral Load Testing:
Among patients on antiretroviral therapy, HIV viral load is the most important marker of treatment response. All patients should have their viral load measured at diagnosis and regularly thereafter. Increased or detectable viral loads in a patient on antiretroviral therapy indicates either nonadherence (most common) or development of drug resistance.
An optimal response to treatment would be a reduction in the viral load to undetectable levels. For the purpose of monitoring therapy, virologic failure is defined as a confirmed viral load in excess of 200 copies/mL. In general, HIV viral load testing should be performed every 3 to 6 months in people living with HIV infection.(11)
HIV-1 Drug Resistance and Susceptibility Testing The global scale-up of ART has led to dramatic reductions in HIV-1 mortality and incidence. However, HIV drug resistance poses a potential threat to the long-term success of ART and is emerging as a threat to the elimination of AIDS as a public health problem. Traditionally, monitoring a patient’s viral load has been done to evaluate the effectiveness of treatments. Increasing viral loads indicate that the virus may have mutated and that a patient’s current regimen is no longer effective at suppressing the virus. Once the virus has mutated and drug resistance develops, a person generally must change medications as different drugs will be needed to keep the virus from multiplying.
Standard genotypic resistance testing (SGRT) is recommended in patients with virological failure when the viral load is > 1000 copies/mL.(12)
HIV-1 drug resistance testing can be performed phenotypically or genotypically. HIV phenotypic antiretroviral susceptibility testing is used to investigate the growing ability of HIV in the presence of antiretroviral drugs through clinical cutoffs for HIV-1 phenotypic resistance estimates. Phenotypic testing is performed with actual viral culture, testing the virus’s ability to replicate in the presence of antiretroviral drugs.
In the clinical setting, genotypic resistance testing is more usual than a phenotypic assay, which involves the use of dideoxy terminator Sanger sequencing of protease, reverse transcriptase and integrase to identify established clinically significant drug resistance mutations (DRMs).(12)
NGS (Next Generation Sequencing) in HIV Testing and Monitoring:
The US FDA recently approved an assay to detect HIV-1 drug resistance mutations using NGS technology. The assay (Sentosa SQ HIV-1 Genotyping Assay) detects HIV-1 drug resistance mutations with >95% sensitivity and specificity in detecting HIV drug resistant mutations. Currently the assay is approved for use only in patients with HIV-1 who are about to start or already taking antiviral therapy and is not intended for diagnosing infection with HIV.(13)
HLA-B*5701 Testing:
Abacavir is a nucleoside analogue reverse transcriptase inhibitor that is associated with severe hypersensitivity reactions in 2%-9% of patients, with those positive for HLA-B*5701 at the greatest risk. The reactions can be lethal. Thus, specific genetic testing for HLA-B*5701 should be performed prior to initiation of abacavir therapy.
OPPORTUNITIES FOR IMPROVEMENT IN DIAGNOSTIC WORKUP IN DIAGNOSTIC WORKUP: FOOD FOR THOUGHT
The latest Ag/Ab combination assays have been very successful screening tools – having reduced the window period and succeeded in identifying 82% of the antibody-negative infections which would otherwise only be detectable by HIV RNA PCR. False-positives have come down to less than 2 per 100,000. But there is always room for improvement.(6)
One suggestion by Branson et al (2019) is to perform a quantitative HIV-1 viral load for every patient with a reactive 4th generation test. This would contribute to confirm the diagnosis and in the future clinical management.(6) In his post in the New England Journal of Medicine Journal Watch blog, Dr. Paul Sax (clinical director of the infectious diseases division at Brigham and Women’s Hospital, Boston, MA) concurs with this view.(14)
A viral load, no matter how low is confirmatory of the diagnosis and treatment can be initiated immediately, which helps in reducing HIV transmission, preserving immune function, and possibly providing the best candidates for HIV cure strategies when they become available in the future.(14)
A negative viral load would indicate the patient is one of the following:
· Patient is an “elite controller”. Elite controllers are a small subset of people living with HIV who have sustained antibody levels but are able to maintain suppressed viral loads for years without antiretroviral therapy (ART).(14,15)
· Patient on antiretroviral therapy who has not disclosed their diagnosis(14)
· Patient is of those rare individuals who is HIV-2 positive(14)
· Patient had a false positive HIV screen(14)
All of these can be further differentiated on doing HIV1/2 antibody differentiation tests.
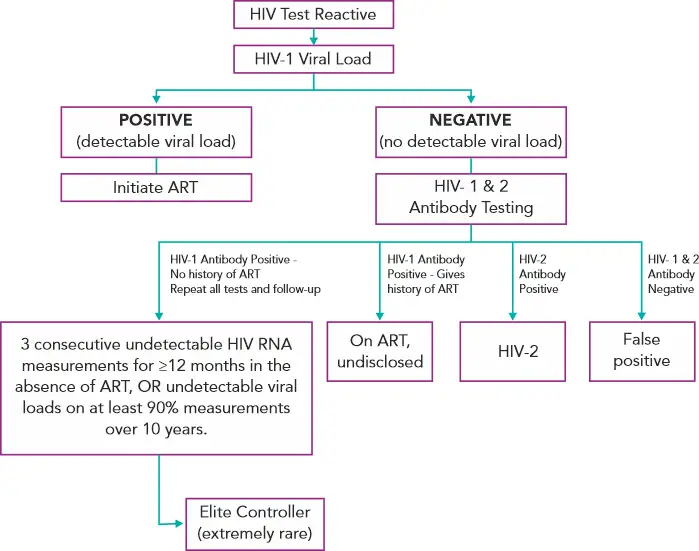
Figure 3 - Suggested Reflex Viral Load Testing Algorithm(6,14,16)
HIV DIAGNOSTIC TESTS AVAILABLE AT SUBURBAN DIAGNOSTICS
Suburban Diagnostics performs HIV antibody/antigen testing using the following methodologies
· Chemiluminescent microparticle immunoassay (CMIA) on the Abbott - Architect i1000SR: This is a 4th generation test detecting HIV p24 antigen and antibodies to HIV type 1 (HIV-1 group M and group O) and/or type 2 (HIV-2).
· Electrochemiluminescence Immunoassay (ECLIA) on the Roche Cobas e411: This is a 4th generation test detecting HIV p24 antigen and antibodies to HIV type 1 (HIV-1 group M and group O) and/or type 2 (HIV-2).
· Immunofiltration: This is a 3rd generation rapid screening test detecting antibodies to HIV-1/2.
· Immunochromatography: This is a 3rd generation rapid screening test detecting antibodies to HIV-1/2.
HIV-RNA PCR FOR DIAGNOSIS
On the molecular front, for HIV-RNA, Suburban Diagnostics uses the fully automated FDA approved platforms from Roche - The Cobas Ampliprep / Cobas Taqman HIV-1 test, Version 2.0 for HIV-1 RNA monitoring of patients infected with group M & group O HIV -1 virus. The two targets (HIV-1 gag & LTR) specific dual labelled probes ensures a complete coverage of Group M subtypes as well as Group O of HIV-1 RNA.
Suburban Diagnostics also offers molecular assays for drug resistance testing with panels for resistance against NRTI and NNRTI drugs.
So, take the first step towards having a healthier you and do blood test at home.
REFERENCES
1. Eberle J, Gürtler L. HIV Types, Groups, Subtypes and Recombinant Forms: Errors in Replication, Selection Pressure and Quasispecies. Intervirology. 2012;55(2):79–83.
2. Hurt CB, Nelson JAE, Hightow-Weidman LB, Miller WC. Selecting an HIV Test: A Narrative Review for Clinicians and Researchers. Sex Transm Dis. 2017 Dec;44(12):739–46.
3. National Guidelines for HIV Testing [Internet]. National AIDS Control Organization, Ministry of Health & Family Welfare, Government of India; 2015 [cited 2019 Nov 26]. Available from: http://www.naco.gov.in/sites/default/files/National_Guidelines_for_HIV_Testing_21Apr2016.pdf
4. Alexander TS. Human Immunodeficiency Virus Diagnostic Testing: 30 Years of Evolution. Clin Vaccine Immunol. 2016 Apr 1;23(4):249–53.
5. Blog LBP. Best Practices for HIV-1/2 Screening: When to Test and What to Test [Internet]. Laboratory Best Practice Blog. [cited 2019 Nov 28]. Available from: https://blog.ucdmc.ucdavis.edu/labbestpractice/index.php/2017/09/15/best-practices-for-hiv-12-screening-when-to-test-and-what-to-test/
6. Branson BM. HIV Diagnostics: Current Recommendations and Opportunities for Improvement. Infect Dis Clin. 2019 Sep 1;33(3):611–28.
7. A “fifth generation” HIV assay? [Internet]. Medical Laboratory Observer. [cited 2019 Nov 26]. Available from: https://www.mlo-online.com/home/article/13008643/a-fifth-generation-hiv-assay
8. Read JS, and the Committee on Pediatric AIDS. Diagnosis of HIV-1 Infection in Children Younger Than 18 Months in the United States. PEDIATRICS. 2007 Dec 1;120(6):e1547–62.
9. Justiz Vaillant AA, Naik R. HIV-1 Associated Opportunistic Infections. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 [cited 2021 Dec 9]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK539787/
10. Guidelines for the Prevention and Treatment of Opportunistic Infections in Adults and Adolescents. :494.
11. DHHS Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV. CDC;
12. Zhao J, Chang L, Wang L. Nucleic acid testing and molecular characterization of HIV infections. Eur J Clin Microbiol Infect Dis. 2019 May;38(5):829–42.
13. Commissioner O of the. FDA authorizes marketing of first next-generation sequencing test for detecting HIV-1 drug resistance mutations [Internet]. FDA. FDA; 2020 [cited 2021 Dec 8]. Available from: https://www.fda.gov/news-events/press-announcements/fda-authorizes-marketing-first-next-generation-sequencing-test-detecting-hiv-1-drug-resistance
14. Our HIV Testing Algorithm Has a Major Problem - Here’s How to Fix It [Internet]. HIV and ID Observations. 2019 [cited 2019 Nov 27]. Available from: https://blogs.jwatch.org/hiv-id-observations/index.php/our-hiv-testing-algorithm-is-broken-heres-how-to-fix-it/2019/10/07/
15. Elite Controllers Definition [Internet]. AIDSinfo. [cited 2019 Nov 27]. Available from: https://aidsinfo.nih.gov/understanding-hiv-aids/glossary/3127/elite-controllers
16. Considerations for the clinical management of HIV elite controllers | The Ontario HIV Treatment Network [Internet]. [cited 2019 Nov 27]. Available from: https://www.ohtn.on.ca/rapid-response-considerations-for-the-clinical-management-of-hiv-elite-controllers/
Want to book a test? Fill up the details & get a callback
Most Viewed
Premarital Health Screening
- 20 Min Read
Typhoid - Signs and Symptoms
- 3 Min Read
Home Isolation Guidelines - Covid-19 Care
- 5 Min Read
HLA B27 Detection: Flow Cytometry & PCR
- 1 Min Read